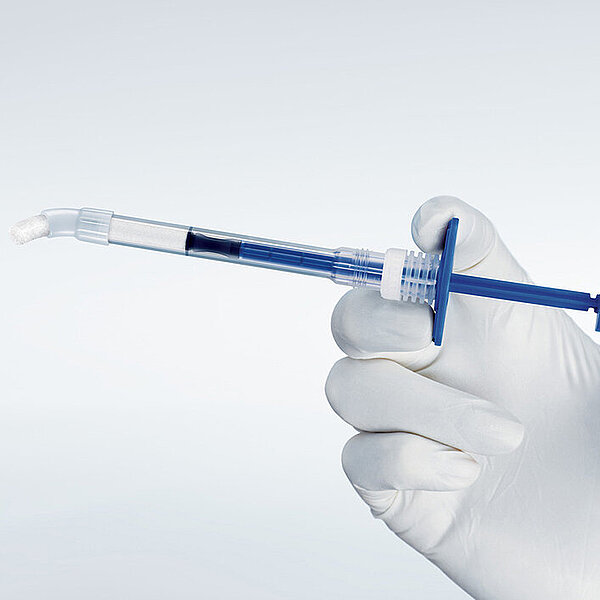
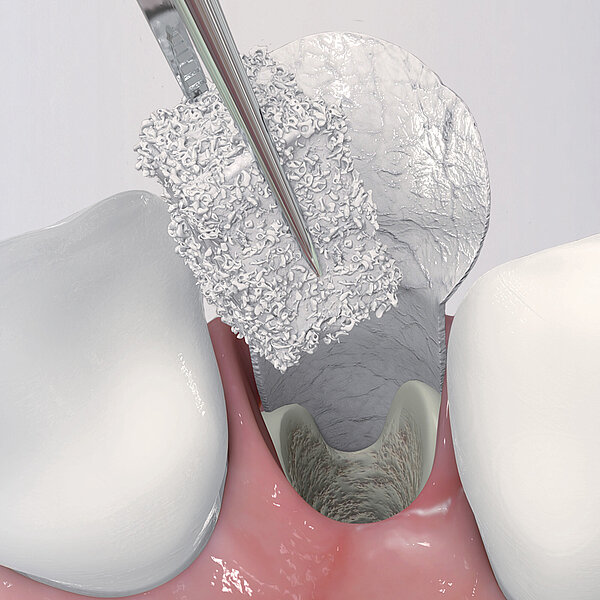
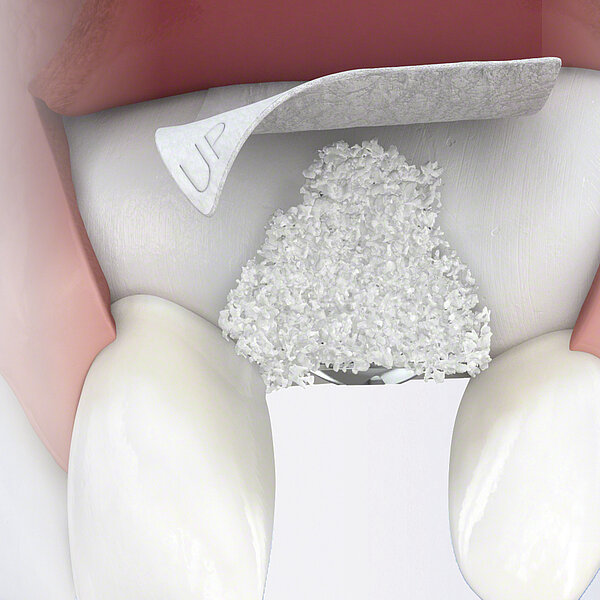
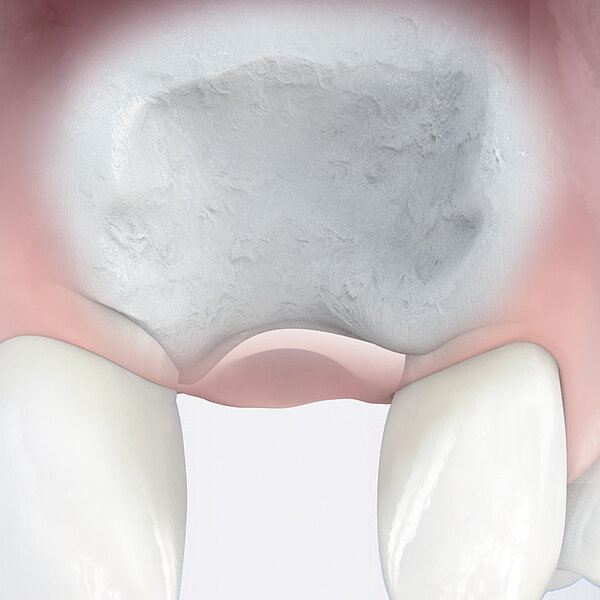
Geistlich Bio-Oss Pen®
GBR이 간편해집니다.
환자의 의료 및 치과 병력, 또는 골 결손 부위의 위치와 같은 요소들은 치과 재생 치료를 복잡하게 만들 수 있는 요인 중 하나입니다. 최근에는 치료 시간 단축, 또는 빠르고 통증 없는 치료에 대한 환자들의 요구도 증가하고 있습니다.
그것이 저희가 Geistlich Bio-Oss Pen을 개발한 이유입니다.
Geistlich Bio-Oss Pen® 은 전세계 재생 시장의 No.1 제품인 Geistlich Bio-Oss® 입자들로 채워진 프리필드 시린지 형태의 제품입니다.4,5,6,7,8,9,10 –또한 전 세계 1,500만명의 환자들에게 사용되었습니다.3
References:
- Data on file. Usability test Geistlich Bio-Oss Pen® 2021
- Karmon B et al.: Int J Periodontics Restorative Dent 2020 40 (2), 223-30.¨
- Data on file. Based on the number of units currently sold. (Wolhusen, Switzerland).
- US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Published in January 2019 by iData Research Inc., 2019. (Market Research).
- South Korea market report suite for dental bone graft substitutes and other biomaterials, iDATA_SKDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Japan market report suite for dental bone graft substitutes and other biomaterials, iDATA_JPDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Published in July 2019 by iData Research Inc., 2019. (Market Research).
- India market report suite for dental bone graft substitutes and other biomaterials, iDATA_INDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Australia market report suite for dental bone graft substitutes and other biomaterials, iDATA_AUDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Data on file. Geistlich Pharma AG usability test Gesitlich Bio-Oss® Pen, 2011
- Galindo-Moreno P et al.: Clin Oral Implants Res 2010; 21 (2), 221-7.
- Orsini G et al.: Oral Dis 2007; 13 (6), 586-93.
- Jung RE et al.: Clin Oral Implants Res 2013; 24 (10), 1065-73.
- Chackartchi T et al.: Clin Oral Implants Res 2011.
- Dos Anjos TL et al.: Int J Oral Maxillofac Surg 2016; 45 (12), 1556-63.
- Urban IA et al.: Int J Periodontics Restorative Dent 2013; 33 (3), 299-307.
- Chiapasco M et al.: Minerva Stomatol 2013; 62 (1-2), 3-16.
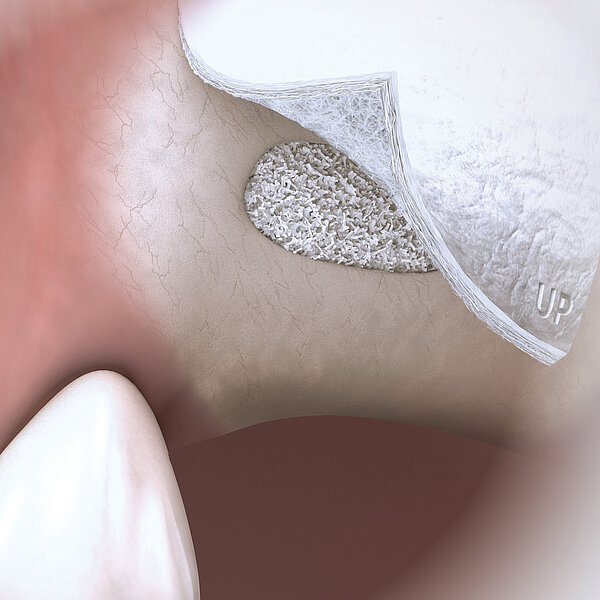
- 사용자에 맞춘 단순한 디자인 : 준비 과정부터 사용 시점까지 Geistlich Bio-Oss Pen® 은 재생 치료를 더욱 쉽게 만들어줍니다.1
- 불가능을 극복 : Geistlich Bio-Oss Pen®의 어플리케이터 팁을 사용하여 구강 내 접근하기 어려운 부위에도 손쉽게 이식재를 적용할 수 있습니다.11
- 정확하고 세밀한 컨트롤: Pen 어플리케이터의 디자인은Geistlich Bio-Oss®의 입자 배출량을 세밀하게 제어할 수 있도록 도와드립니다.1
- 장기간 유지되는 결과물 : Geistlich Bio-Oss®는 세계 최고의 생체 기능성 재생물질로 혈관화와 신생골 형성을 돕습니다. 12 특유의 느리고 낮은 흡수율은 이식재의 안정성을 증대시켜13 임플란트의 장기 생존율에 있어 최고의 결과물을 보여줍니다. 14
References:
- Data on file. Usability test Geistlich Bio-Oss Pen® 2021
- Karmon B et al.: Int J Periodontics Restorative Dent 2020 40 (2), 223-30.¨
- Data on file. Based on the number of units currently sold. (Wolhusen, Switzerland).
- US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Published in January 2019 by iData Research Inc., 2019. (Market Research).
- South Korea market report suite for dental bone graft substitutes and other biomaterials, iDATA_SKDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Japan market report suite for dental bone graft substitutes and other biomaterials, iDATA_JPDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Published in July 2019 by iData Research Inc., 2019. (Market Research).
- India market report suite for dental bone graft substitutes and other biomaterials, iDATA_INDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Australia market report suite for dental bone graft substitutes and other biomaterials, iDATA_AUDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Data on file. Geistlich Pharma AG usability test Gesitlich Bio-Oss® Pen, 2011
- Galindo-Moreno P et al.: Clin Oral Implants Res 2010; 21 (2), 221-7.
- Orsini G et al.: Oral Dis 2007; 13 (6), 586-93.
- Jung RE et al.: Clin Oral Implants Res 2013; 24 (10), 1065-73.
- Chackartchi T et al.: Clin Oral Implants Res 2011.
- Dos Anjos TL et al.: Int J Oral Maxillofac Surg 2016; 45 (12), 1556-63.
- Urban IA et al.: Int J Periodontics Restorative Dent 2013; 33 (3), 299-307.
- Chiapasco M et al.: Minerva Stomatol 2013; 62 (1-2), 3-16.
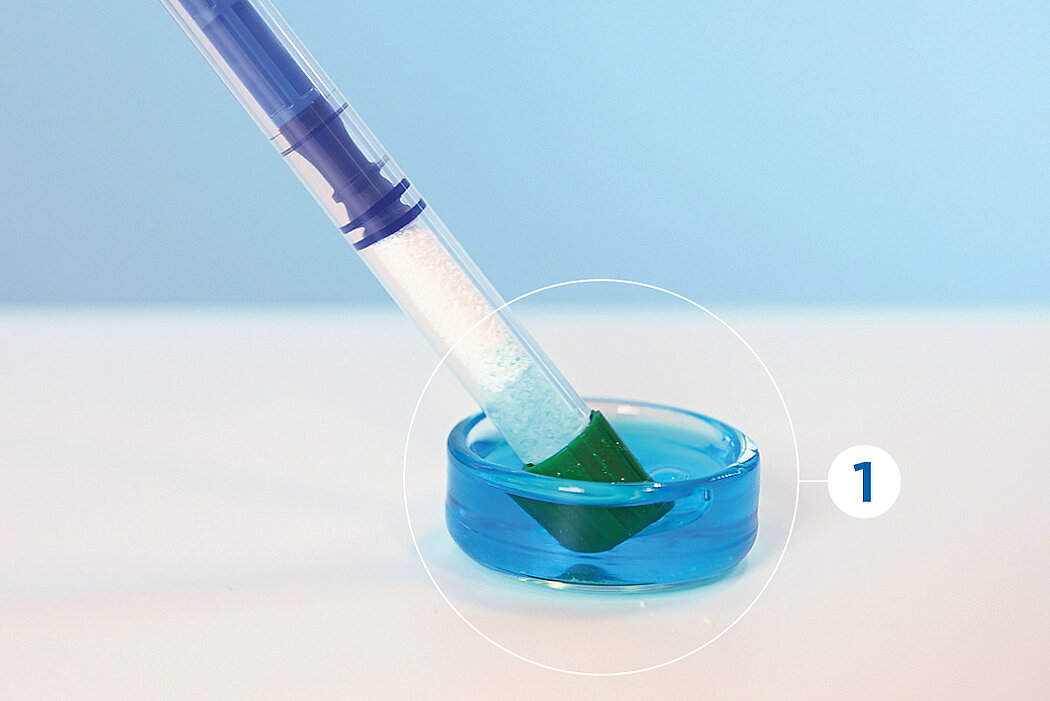
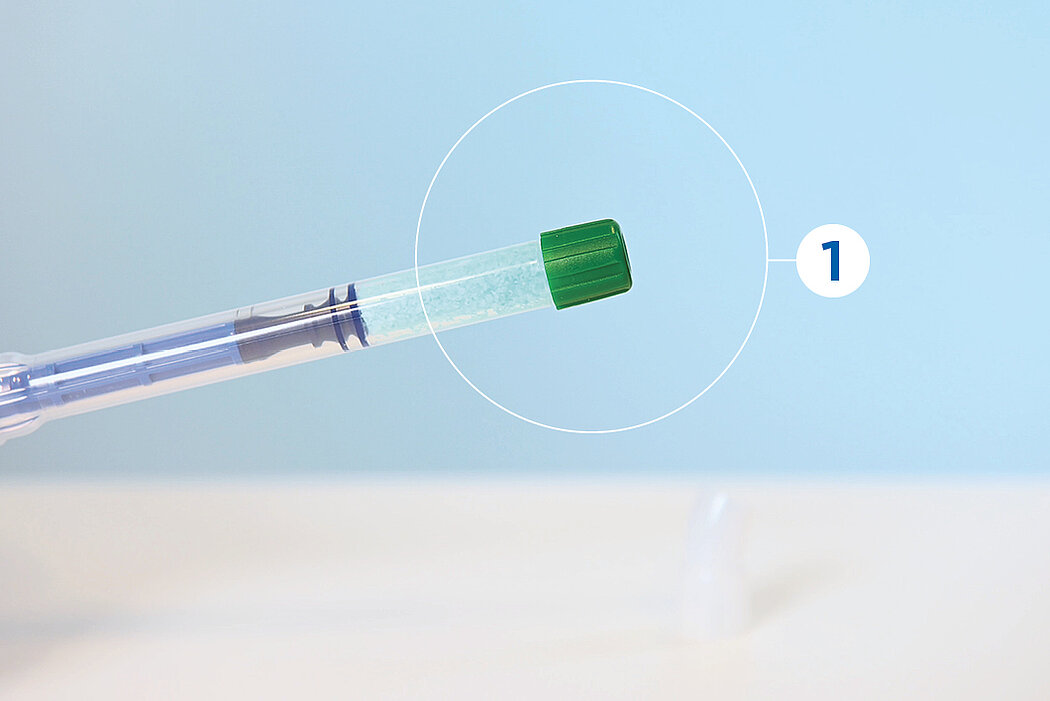
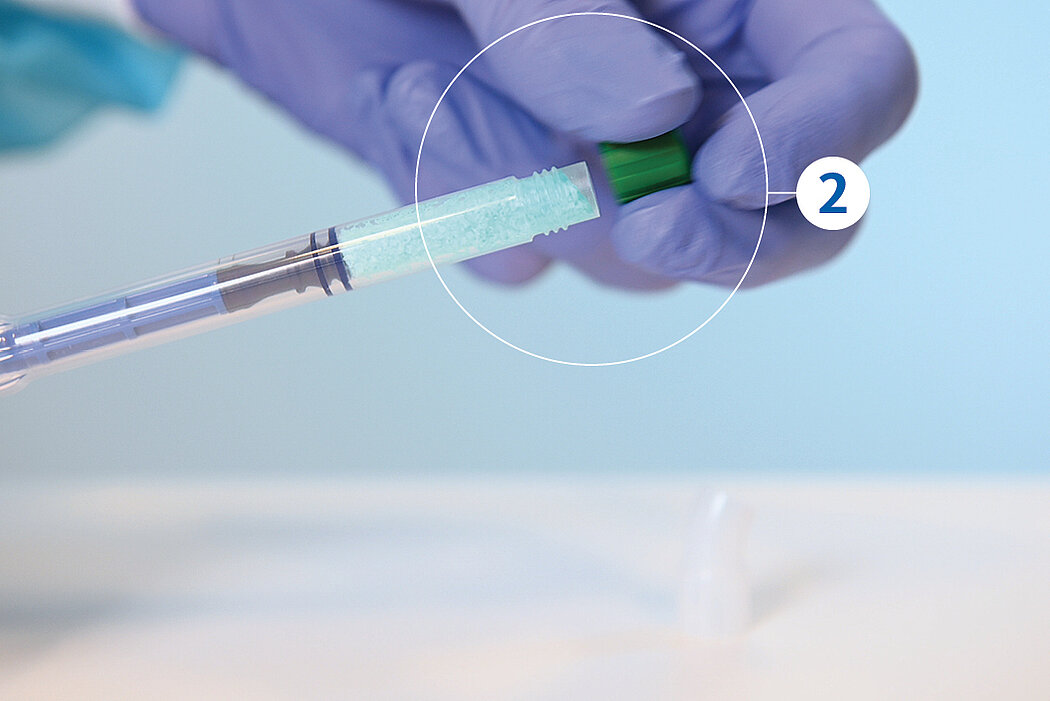
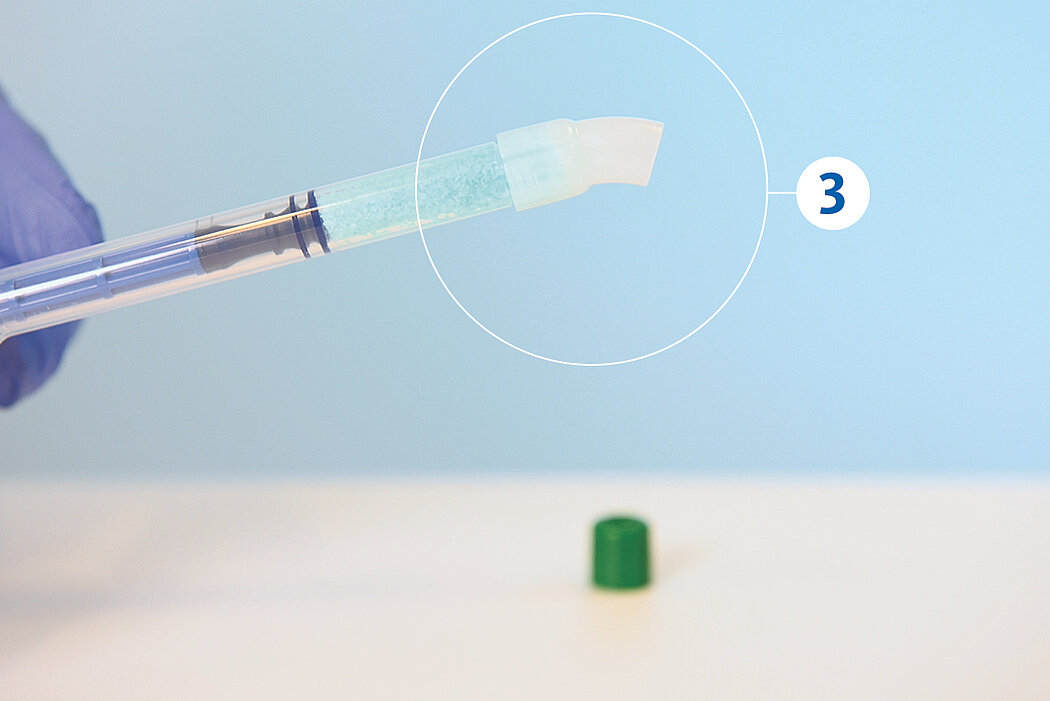
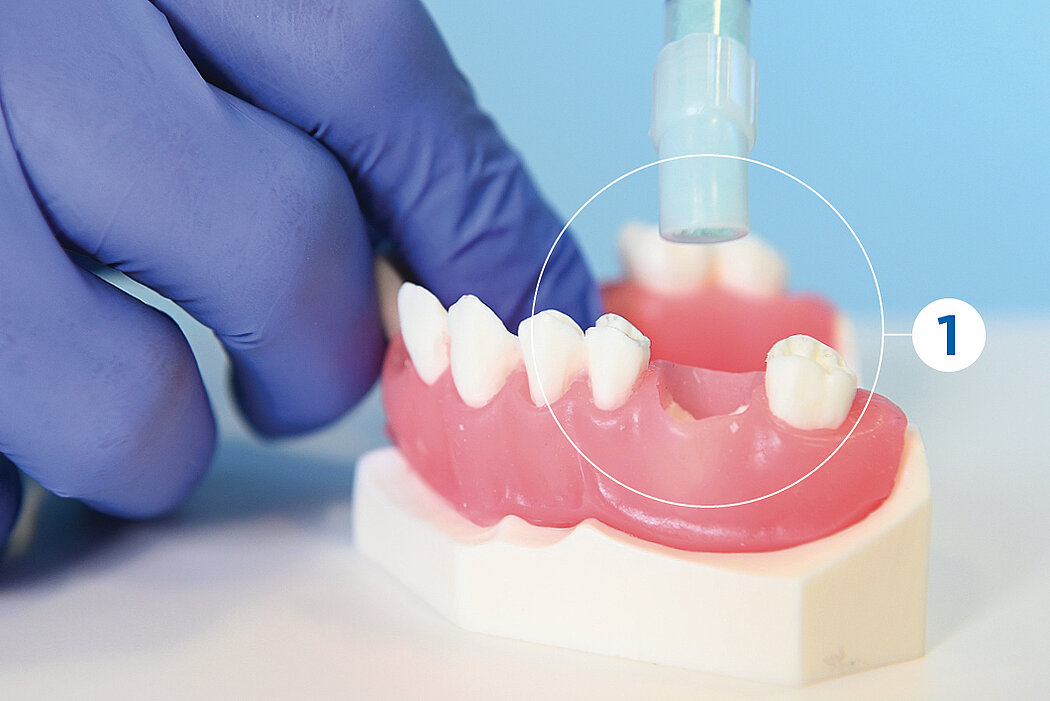
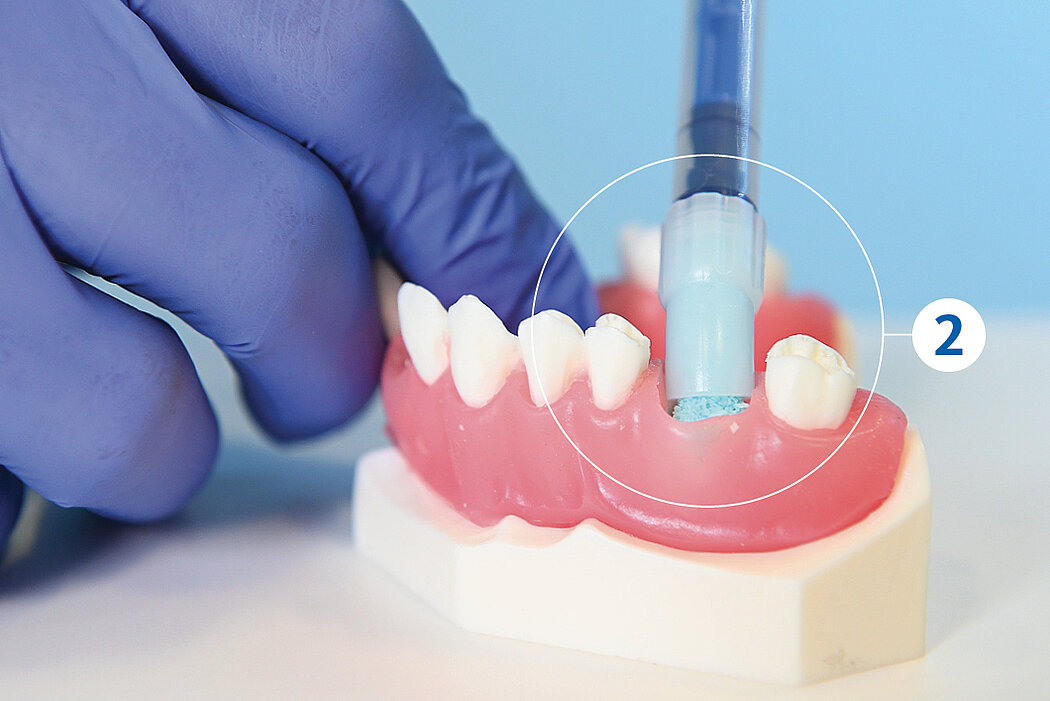
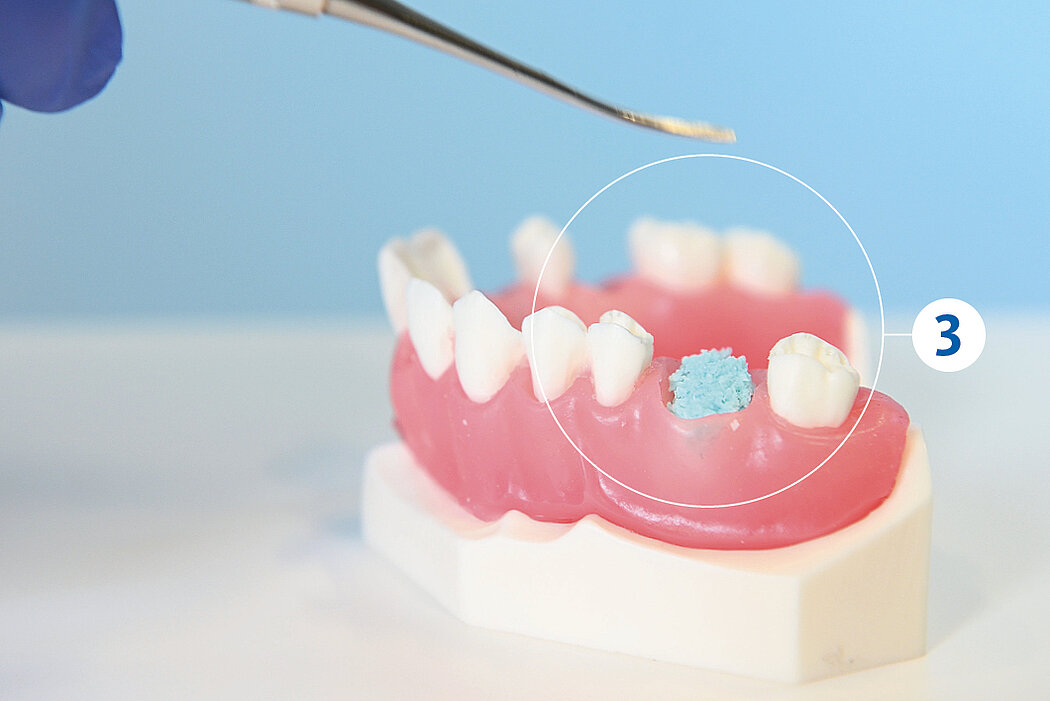
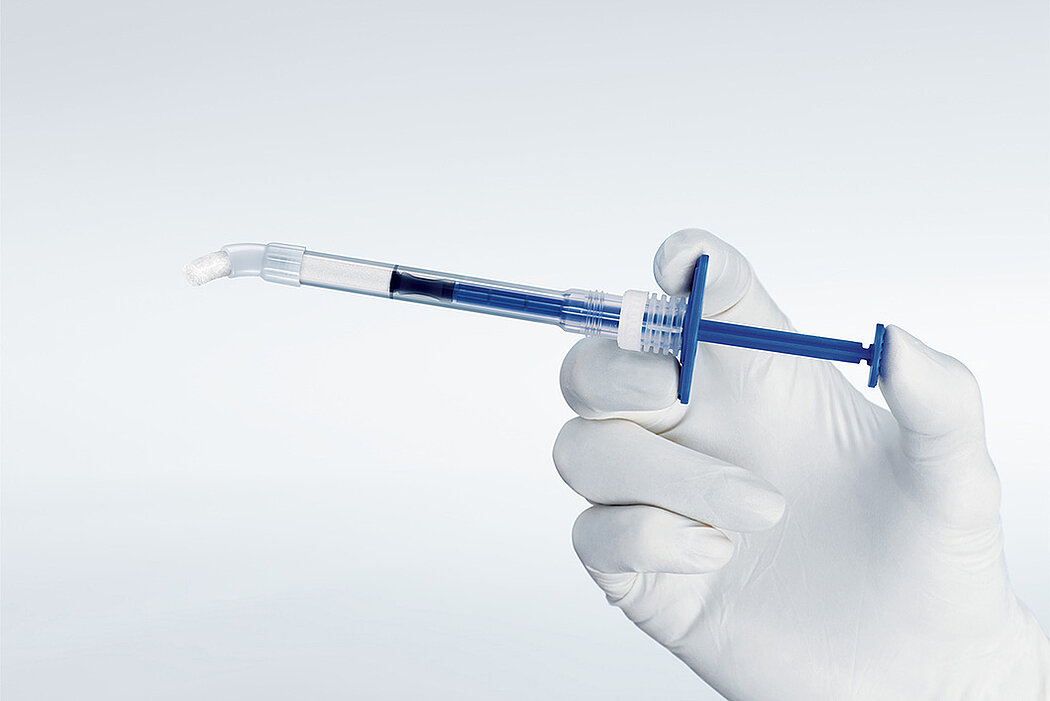
간단한 3단계로 준비 완료 : 수화. 팁 교체. 적용
간단한 몇 가지 단계만 거치면 Geistlich Bio-Oss® 를 사용할 준비가 완료되며, 골 결손부에 바로 적용할 수 있습니다.
References:
- Data on file. Usability test Geistlich Bio-Oss Pen® 2021
- Karmon B et al.: Int J Periodontics Restorative Dent 2020 40 (2), 223-30.¨
- Data on file. Based on the number of units currently sold. (Wolhusen, Switzerland).
- US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Published in January 2019 by iData Research Inc., 2019. (Market Research).
- South Korea market report suite for dental bone graft substitutes and other biomaterials, iDATA_SKDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Japan market report suite for dental bone graft substitutes and other biomaterials, iDATA_JPDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Published in July 2019 by iData Research Inc., 2019. (Market Research).
- India market report suite for dental bone graft substitutes and other biomaterials, iDATA_INDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Australia market report suite for dental bone graft substitutes and other biomaterials, iDATA_AUDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Data on file. Geistlich Pharma AG usability test Gesitlich Bio-Oss® Pen, 2011
- Galindo-Moreno P et al.: Clin Oral Implants Res 2010; 21 (2), 221-7.
- Orsini G et al.: Oral Dis 2007; 13 (6), 586-93.
- Jung RE et al.: Clin Oral Implants Res 2013; 24 (10), 1065-73.
- Chackartchi T et al.: Clin Oral Implants Res 2011.
- Dos Anjos TL et al.: Int J Oral Maxillofac Surg 2016; 45 (12), 1556-63.
- Urban IA et al.: Int J Periodontics Restorative Dent 2013; 33 (3), 299-307.
- Chiapasco M et al.: Minerva Stomatol 2013; 62 (1-2), 3-16.
여러분의 선택이 남았습니다.
Geistlich Bio-Oss Pen®은다음과 같은 골유도재생술 및 세포유도재생술에 사용되도록 디자인되었습니다.
- 치조능 골 결손
- 악안면 이상 및 기형
Geistlich Bio-Oss Pen® 은 2가지의 Geistlich Bio-Oss® 입자 사이즈로 제공됩니다.
두가지 입자 사이즈의 차이는 부피에 있습니다. 또한 작거나 큰 입자로 동일한 임상 결과를 얻을 수 있습니다.15,16
재생 관련 상황에 따라 가장 적합한 입자 크기와 용량을 자유롭게 선택하실 수 있으나, 저희는 아래 사항을 권장합니다.
References:
- Data on file. Usability test Geistlich Bio-Oss Pen® 2021
- Karmon B et al.: Int J Periodontics Restorative Dent 2020 40 (2), 223-30.¨
- Data on file. Based on the number of units currently sold. (Wolhusen, Switzerland).
- US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Published in January 2019 by iData Research Inc., 2019. (Market Research).
- South Korea market report suite for dental bone graft substitutes and other biomaterials, iDATA_SKDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Japan market report suite for dental bone graft substitutes and other biomaterials, iDATA_JPDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Published in July 2019 by iData Research Inc., 2019. (Market Research).
- India market report suite for dental bone graft substitutes and other biomaterials, iDATA_INDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Australia market report suite for dental bone graft substitutes and other biomaterials, iDATA_AUDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Data on file. Geistlich Pharma AG usability test Gesitlich Bio-Oss® Pen, 2011
- Galindo-Moreno P et al.: Clin Oral Implants Res 2010; 21 (2), 221-7.
- Orsini G et al.: Oral Dis 2007; 13 (6), 586-93.
- Jung RE et al.: Clin Oral Implants Res 2013; 24 (10), 1065-73.
- Chackartchi T et al.: Clin Oral Implants Res 2011.
- Dos Anjos TL et al.: Int J Oral Maxillofac Surg 2016; 45 (12), 1556-63.
- Urban IA et al.: Int J Periodontics Restorative Dent 2013; 33 (3), 299-307.
- Chiapasco M et al.: Minerva Stomatol 2013; 62 (1-2), 3-16.
간편함을 경험해보세요.
30년 이상의 기간 동안 입증된 Geistlich Bio-Oss® 골 이식재의 재생 성능과 사용 편의성11 덕에 세계적인 임상가들은 이미 Geistlich Bio-Oss Pen®을 사용하고 있습니다.14, 17, 18
References:
- Data on file. Usability test Geistlich Bio-Oss Pen® 2021
- Karmon B et al.: Int J Periodontics Restorative Dent 2020 40 (2), 223-30.¨
- Data on file. Based on the number of units currently sold. (Wolhusen, Switzerland).
- US market report suite for dental bone graft substitutes and other biomaterials, iDATA_USDBGS19_MS, Published in January 2019 by iData Research Inc., 2019. (Market Research).
- South Korea market report suite for dental bone graft substitutes and other biomaterials, iDATA_SKDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Japan market report suite for dental bone graft substitutes and other biomaterials, iDATA_JPDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Europe market report suite for dental bone graft substitutes and other biomaterials, iDATA_EUDBGS19_MS, Published in July 2019 by iData Research Inc., 2019. (Market Research).
- India market report suite for dental bone graft substitutes and other biomaterials, iDATA_INDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Australia market report suite for dental bone graft substitutes and other biomaterials, iDATA_AUDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- China market report suite for dental bone graft substitutes and other biomaterials, iDATA_CHDBGS18_MS, Published in November 2018 by iData Research Inc., 2018. (Market Research).
- Data on file. Geistlich Pharma AG usability test Gesitlich Bio-Oss® Pen, 2011
- Galindo-Moreno P et al.: Clin Oral Implants Res 2010; 21 (2), 221-7.
- Orsini G et al.: Oral Dis 2007; 13 (6), 586-93.
- Jung RE et al.: Clin Oral Implants Res 2013; 24 (10), 1065-73.
- Chackartchi T et al.: Clin Oral Implants Res 2011.
- Dos Anjos TL et al.: Int J Oral Maxillofac Surg 2016; 45 (12), 1556-63.
- Urban IA et al.: Int J Periodontics Restorative Dent 2013; 33 (3), 299-307.
- Chiapasco M et al.: Minerva Stomatol 2013; 62 (1-2), 3-16.
이제 그 간편함을 여러분이 경험해 보실 차례입니다.